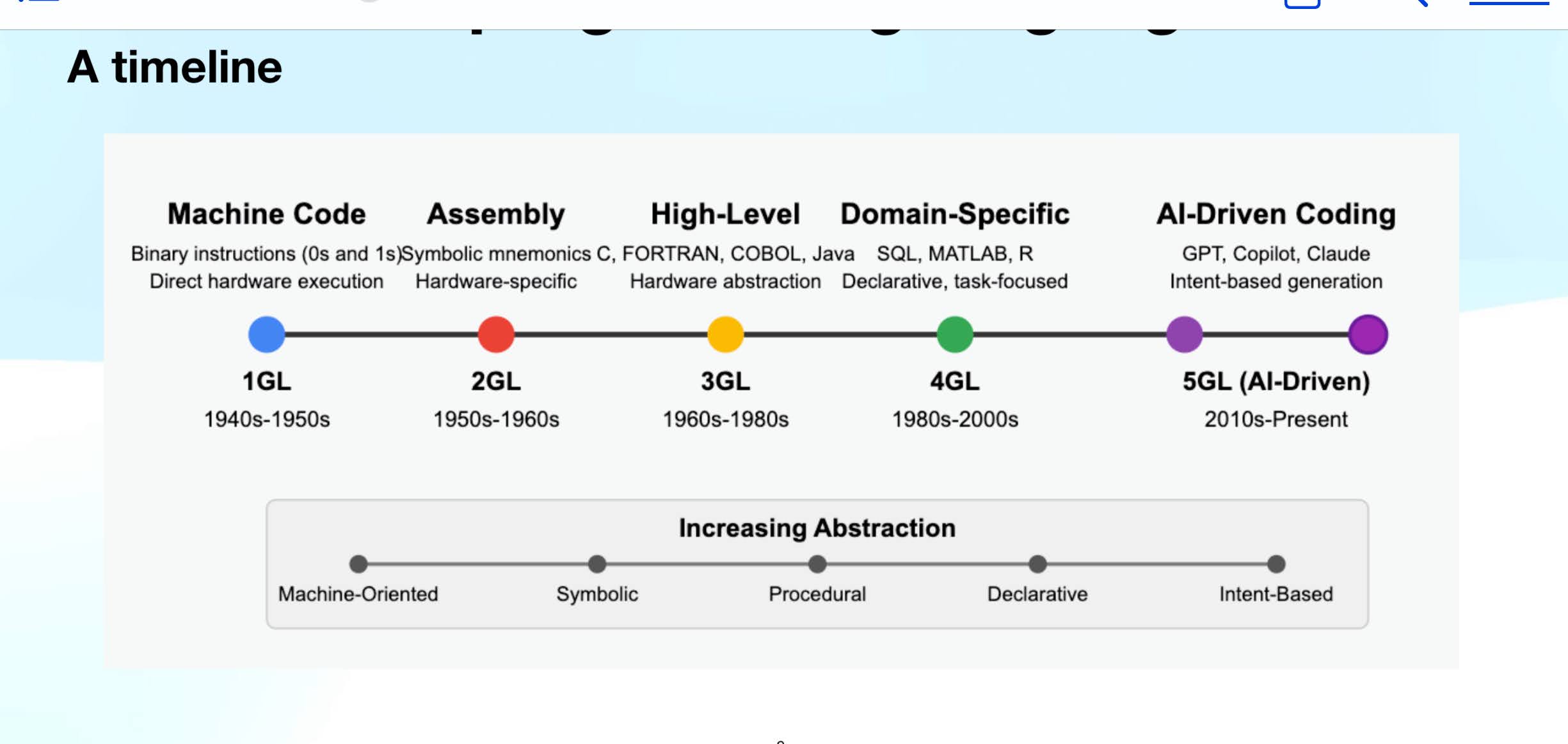
"GCSE Chemistry Revision: Unveiling the Mysteries of Quantitative Chemistry for...
Unveiling the Mysteries of Quantitative Chemistry for Exam Success
Understanding Quantitative Chemistry
Quantitative chemistry is a crucial part of the GCSE Chemistry curriculum, focusing on the measurement and calculation of chemical quantities. Mastering this topic is essential for exam success.
Key Concepts in Quantitative Chemistry
- Mole Concept: Understanding the mole as a unit of measurement for amount of substance.
- Avogadro's Number: The number of atoms, ions, or molecules in one mole of a substance, approximately 6.022 x 1023.
- Empirical and Molecular Formulas: Determining the simplest ratio of elements in a compound and the actual number of atoms of each element in a molecule.
- Stoichiometry: Calculating the quantities of reactants and products in chemical reactions.
- Concentration: Understanding solutions and calculating concentration in terms of molarity.
Tips for Exam Success
- Practice Calculations: Regularly solve problems related to moles, stoichiometry, and concentration to build confidence.
- Use Dimensional Analysis: This technique helps in converting units and solving complex problems systematically.
- Memorize Key Formulas: Ensure you know essential formulas, such as those for calculating moles and concentration.
- Understand Rather Than Memorize: Focus on understanding the concepts behind the calculations to apply them effectively in different scenarios.
- Review Past Papers: Practice with past exam papers to familiarize yourself with the question format and time management.
Additional Resources
For further study, consider exploring online resources and textbooks that offer detailed explanations and practice problems. Engaging with interactive simulations can also enhance your understanding of quantitative chemistry concepts.
Browse Categories 📚
📖 AI Case Studies
📖 AI Certification
📖 AI Certification & Career Development
📖 AI Certification & Professional Development
📖 AI Certification and Dataset Management
📖 AI Certification and Deployment
📖 AI Certification and Skills Development
📖 AI Certification and Training
📖 AI Certification and Trends
📖 AI Dataset Management
📖 AI Development with Python
📖 AI Ethics and Compliance
📖 AI Ethics and Governance
📖 AI Ethics and Responsible AI
📖 AI Model Evaluation
📖 AI Model Implementation
📖 AI Model Optimization
📖 AI Trends and Innovations
📖 AI/ML Certification
📖 AI/ML Data Management
📖 AI/ML Model Selection
📖 AI/ML Trends
📖 Biology Education
📖 Chemistry Education
📖 Chemistry Revision
📖 Cloud AI Infrastructure
📖 Computer Vision Applications
📖 Conversational AI Development
📖 Currency Exchange
📖 Data Mining & Visualization
📖 Data Preprocessing
📖 Data Science and Visualization
📖 Data Visualization
💻 Digital Tools
📖 Economics Education
📖 Economics Revision
📖 Edge AI & IoT
📖 Education
📖 Education Technology
📖 Education and Curriculum Development
📖 Education and Parenting
📖 Education and Study Techniques
📖 Education and Technology
📖 Educational Strategies
📖 Educational Technology
📖 Educational Technology in Biology
📖 Educational Technology in Chemistry
📖 Educational Technology in Mathematics
📖 Educational Technology in Physics
📖 Environmental Science
📖 Ethical AI Development
🎯 Exam Preparation
📖 Feature Engineering
📖 Feature Engineering & Model Optimization
📖 Financial Literacy
📖 GCSE Biology
📖 GCSE Biology Revision
📖 GCSE Chemistry Revision
📖 GCSE Economics Revision
📖 GCSE Exams & Assessment
📖 GCSE Maths Revision
📖 GCSE Maths Skills
📖 GCSE Physics
📖 GCSE Physics Revision
📖 GCSE Study Skills
📚 GCSE Subjects
📖 GPU Architecture & Optimization
💡 General Tips
📖 Generative AI Certification and Applications
📖 LLM Applications in Industry
📖 LLM Training & Deployment
📖 MLOps & Model Deployment
📖 Machine Learning
📖 Machine Learning Certification
📖 Machine Learning Engineering
📖 Machine Learning Implementation
📖 Machine Learning Techniques
📖 Math Skills
📖 Math in Everyday Life
📖 Mathematics
📖 Mathematics Education
📖 Mathematics Fundamentals
📖 Mathematics Revision
📖 Mathematics in Everyday Life
📖 Mental Health and Education
📖 Model Deployment & Reliability
📖 Model Evaluation & Validation
📖 Model Interpretability
📖 Modern Genetics and Biotechnology
📖 NVIDIA AI Certification
📖 Natural Language Processing
👨👩👧👦 Parent Support
📖 Parental Guidance
📖 Personal Finance Basics
📖 Physics Education
📖 Practical Math Skills
📖 Responsible AI & Certification
📖 Retrieval-Augmented Generation (RAG)
📖 Science Education
📖 Student Finance
🧠 Student Wellbeing
📖 Study Skills
📖 Study Skills & Exam Preparation
⚡ Study Techniques
Ready to boost your learning? Explore our comprehensive resources above, or visit TRH Learning to start your personalized study journey today!
📚
Category: GCSE Chemistry Revision
Last updated: 2025-09-24 09:55 UTC